Portrait Prof. Dr. Birgit Esser
Image: Prof. Dr. Birgit EsserCurriculum Vitae
Birgit Esser studied Chemistry at the Universities of Heidelberg, Germany, and Bristol, UK, and obtained her PhD from the University of Heidelberg under the supervision of Prof. Rolf Gleiter in 2008. After a postdoctoral stay in the group of Timothy M. Swager at MIT she started her independent career in 2012 at the University of Bonn. Since 2015, she is professor for Molecular/Organic Functional Materials at the University of Freiburg. Her scientific interests include the design and synthesis of electrode materials for organic batteries, conjugated small molecules and polymers for optoelectronics, supramolecular donor-acceptor interactions as well as the synthesis and investigation of cyclic conjugated molecules.
Abstract
(Hetero)aromatic Redox Polymers as Electrode-Active Materials for Organic Batteries
Prof. Dr. Birgit Esser
Institute for Organic Chemistry and Freiburg Materials Research Center
University of Freiburg
E-mail: besser@oc.uni-freiburg.de
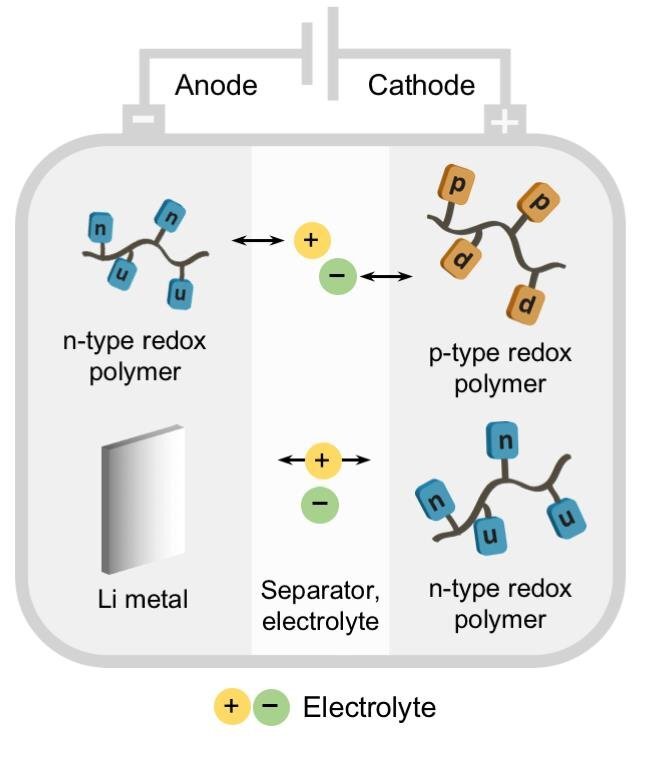
In face of the climate change there is a strong and growing demand for the storage of renewable energies. Reliable electricity storage devices such as batteries and electrochemical capacitors are required.[1] Organic electrode materials have attracted great interest, as they can be prepared from renewable, sustainable or less-limited resources, they are easy to recycle as well as potentially safer and cheaper to produce, leading to a low carbon footprint.[2] A promising class of organic electrode materials are redox polymers – polymers containing groups that can be reversibly reduced or oxidized.[3] In this talk organic redox polymers will be presented containing π-systems as redox-active functionalities.[4–8] The synthesis and electrochemical properties of these polymers will be discussed as well as their application as electrode-active materials in batteries.
Graphic on the abstract
Image: Prof. Dr. Birgit EsserReferences:
- D. Lindley, Nature 2010, 463, 18-20.
- a) P. Poizot, F. Dolhem, Energy Environ. Sci. 2011, 4, 2003-2019;
b) Y. Liang, Z. Tao, J. Chen, Adv. Energy Mater. 2012, 2, 742–769;
c) Z. Song, H. Zhou, Energy Environ. Sci. 2013, 6, 2280–2301;
d) T. B. Schon, B. T. McAllister, P.-F. Li, D. S. Seferos, Chem. Soc. Rev. 2016, 45, 6345–6404. - S. Muench, A. Wild, C. Friebe, B. Häupler, T. Janoschka, U. S. Schubert, Chem. Rev. 2016, 116, 9438–9484.
- M. E. Speer, M. Kolek, J. J. Jassoy, J. Heine, M. Winter, P. M. Bieker, B. Esser, Chem. Commun. 2015, 51, 15261– 15264.
- M. Kolek, F. Otteny, P. Schmidt, C. Mück-Lichtenfeld, C. Einholz, J. Becking, E. Schleicher, M. Winter, P. Bieker, B. Esser, Energy Environ. Sci. 2017, 10, 2334–2341.
- M. E. Speer, C. Sterzenbach, B. Esser, ChemPlusChem 2017, 82, 1274–1281.
- F. Otteny, M. Kolek, J. Becking, M. Winter, P. Bieker, B. Esser, Adv. Energy Mater. 2018, 1802151.
- M. Kolek, F. Otteny, J. Becking, M. Winter, B. Esser, P. Bieker, Chem. Mater. 2018, 30, 6307–6317.