Dr. Masaru Yao
Image: Dr. Masaru YaoCurriculum Vitae
Masaru Yao received Ph.D. in physical organic chemistry at Keio University (Japan) in 2005. During the Ph.D. course, he was engaged in the study of Langmuir-Blodgett film and molecular magnetism, and synthesized various novel heterocyclic compounds. After that he started to study battery materials for use in nickel/metal-hydride batteries, lithium ion batteries, and electric double layer capacitors in the National Institute of Advanced Industrial Science and Technology (AIST). He is now a senior research scientist at the Research Institute of Electrochemical Energy, Department of Energy and Environment, AIST. His research interest includes the design and synthesis of organic materials for use in rechargeable batteries. He has published more than 20 papers in the field of organic battery since 2010 and have reported a dozen of molecule-based active materials.
Abstract
Design for long cycle-life quinone-type active materials by oligomerization
As a candidate category of minor-metal free electrode materials for use in rechargeable lithium batteries, we have focused on a series of low-molecular-weight quinone derivatives [1]. These compounds have high theoretical capacity based on their multi-electron transfer reactions; however, they often show poor cycle-stabilities. One of the reasons for the capacity fade is believed to be the dissolution of redox active molecules into the electrolyte solution.
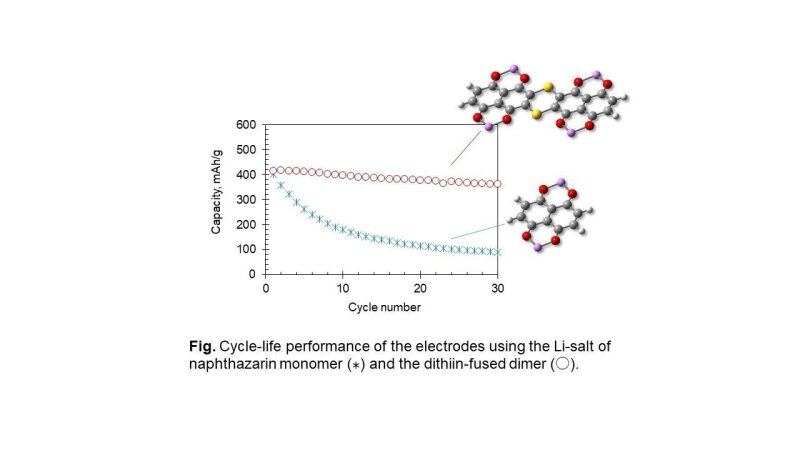
To suppress the dissolution, we found that the oligomerization of the redox active units is an effective way. For example, in the case of the anthraquinone (AQ) derivatives, a fused molecule or acetylene-unit-connected oligomers, which have a lower solubility, exhibits a longer cycle-life than the AQ monomer [2]. A similar result was also observed for the naphthazarin (5,8-dihydroxy-1,4-naphthoquinone) derivatives exhibiting high discharge capacities of about 400 mAh/g [3] as shown in the figure below.
A quantum chemistry calculation revealed that the intermolecular binding energy becomes a few times higher by the oligomerization than that of the monomer. The estimated binding energy values (80-120 kJ/mol) for the oligomers are comparable to the level of the covalent bonding (100-300 kJ/mol), which would be beneficial to suppress the dissolution of the organic active materials into the electrolyte solutions.
Our finding in this study will be a guide to design a novel organic compound showing a high capacity and good cycling performance.
Grafik zum Abstract
Image: Dr. Masaru YaoReferences
- M. Yao, H. Senoh, S. Yamazaki, Z. Siroma, T. Sakai, K. Yasuda, J. Power Sources, 2010, 195, 8336-8340.
- M. Yao, S. Yamazaki, H. Senoh, T. Sakai, T. Kiyobayashi, Mater. Sci. Eng., B, 2012, 177, 483-487.
- M. Yao, H. Ando, T. Kiyobayashi, N. Takeichi, The 57th Battery Symposium in Japan, 2016, #3A09.